| © Bob Heath
published in ANVIL Magazine, March 1998
A symposium on the subject of metals in antiquity was held at Harvard University in Cambridge, Massachusetts last September. It was attended by about 150 archaeologists and scientists. The event was jointly sponsored by Harvard and Bradford University in England. My wife, who is a chemist, saw the advertisement for the event in one of her professional newsletters and suggested that it might be something interesting for me to attend, since I do blacksmithing as a hobby. A Web page address on the Internet was given in the news items and although I am a real novice with a computer, it was fairly easy to acquire the site and log on. A quick review of the program and agenda was convincing enough for a tuition commitment to be sent in, along with a request for airline tickets to Boston and reservations just off campus near the Peabody Museum. The museum is famous for all of the archaeological treasures housed within its walls. The agenda called for a reception in the Maya Room on one of the nights after a round of papers had been presented during the preceding day. It was difficult to resist an invitation to brush shoulders with the very top archaeologists who are working with metals in such surroundings as these. The Peabody Museum has been an enduring source of dreams and fantasies about archaeology for me since learning of its existence in 1958, when a team from Harvard came to Mississippi to excavate a complex of Indian mounds near my home. The archaeologists, led by Dr. Stephen Williams, were not looking for artifacts made of metal, since the Indians down here who built the mounds had very limited access to metal. Only copper was known to have been traded in ancient times here. Native copper, picked up by the Indians in the form of large nuggets from naturally occurring geological outcroppings in the region of the Great Lakes of North America, has been found in Mississippi. It has been beautifully forged into ceremonial objects that are over 500 years old. The winged eagle designs, about the size of one's hand, were forged from thin sheets of beaten copper in a technique called recosso. It has always been puzzling to me, as a worker in metals, how the Indians were able to perform such flawless craftsmanship in this metal with such limited access to the materials. That riddle has never been solved by archaeologists who recover these rare items in the southeastern United States from time to time. The Indians had to know a great deal about the principles of annealing and the working limits of strain hardening to be able to produce these things. The conference orientation was centered around the archaeology of metals as they related to the antiquity of Western Europe and the Middle East/Mediterranean area of the world. There were some papers that dealt with metal objects recovered from historic French and Indian contact sites from the 18th century in Canada, and some interesting studies done of very fine bronze statues and objects from the Far East. The presentations and lectures on metals began with subjects starting around the dawn of the Bronze Age in Mycennian Greece, shifted to Roman methods of smelting iron, and ending with some fascinating studies on metal artifacts recovered from Viking furnace sites and medieval sites in Western Europe. There were a surprising number of people at the symposium who were not archaeologists, and represented the community of professionals who operate physical and geophysical laboratories which perform quantitative and qualitative analyses on samples of metals, ores and slags. These results are a great help to archaeologists who interpret them in an anthropological context. For example, if a laboratory performs a trace element analysis on a bronze arrowhead, in many cases the results can be matched to the same unique patterns of trace elements at the ore source or mine where the metal was extracted from the earth. This approach can be used on iron, tin, copper, lead, and other metals such as gold and silver which were used in antiquity. These methods allow some insight on trade patterns of the various cultures that were extracting, trading, and using the metals. After two days of listening to very interesting lectures, the bulk of the subjects presented seemed to fall into the two basic categories of iron and copper or copper-based metals. Bronze is an alloy of solid solution consisting of a small percentage of tin mixed into copper. About two-thirds of the attendees were interested in copper and the other one-third were interested in iron. The copper people were very pleased to hear about the new excavations and studies on the island of Cypress where the ancients mined copper ore and smelted the metal on the spot. One woman presented a fascinating paper on Mycennian ceramic vases which had been made to resemble golden vessels by first covering them with tin foil and firing them into a golden color. We of the iron persuasion were interested in this subject, but when the presentations of Viking iron furnaces, Roman iron-making methods and African iron smelting were presented, our interests became intense. Three lectures stood out in my mind. The first - and the most outstanding from a practical blacksmithing perspective - was given by Dr. Arne Espelund, who has been involved with the excavations of some 200 Viking-era iron furnace or iron smelting sites located in the mountains in Norway. He described Viking and later Norwegian methods of smelting iron from bog ores and stated that some of the sites he had investigated were in almost untouched, pristine condition after over 1,000 years of being idle. Archaeologists think there are about 300 additional very old furnace sites in Norway that have not been found yet. His investigations indicated that small-scale iron smelting furnaces in Norway had changed little over the past 1,000 years, beginning with early Viking methods and extending down to relatively recent times when Norwegian farmers of the last century smelted iron from bog ores to produce enough metal from which to forge farm implements instead of broad swords, as their ancestors had. Bog ore is a precipitate of iron that has been leeched out of iron-bearing soil through the action of water. When it rains, if there is enough iron in the soil, it can be dissolved into a liquid solution. When the soluble iron runs off as drainage, the flow will sometimes run into bogs or swampy areas where it stagnates as the iron-laden water accumulates. When normal swamp water is present in a bog, it tends to form an acid that results from rotting vegetable matter present in the bog. Tannic acid is one form of acid present as are other chemical conditions that tend to produce an acidic bog water. When the iron-laden runoff from the iron-bearing soil reaches a bog like this, the iron precipitates out due to the action of the bog acid and is deposited in lenses or layers of iron ore on the bottom of the bog. Over hundreds of years of this type of action, enough iron ore can accumulate to produce useful deposits of iron that can be exploited by those who know how to smelt the ore into pure iron. These types of deposits of iron ore are relatively free of undesirable impurities because much of the detrimental elements associated with normal mineral iron ores, such as phosphorous, may not have precipitated in the bog.
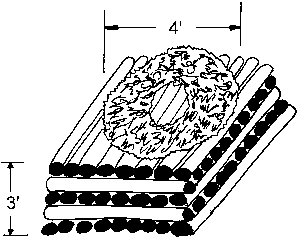
Dr. Espelund described a two-step "direct" iron-smelting process that has been used in Norway for 1,000 years. The first step consists of placing a fourfoot (in diameter) donut-shaped ring of crushed ore on top of layered pine cord wood piled to a height of about three feet. The hole is left in the middle of the ore to facilitate the flow of air into the burning fire. The pile is set on fire and allowed to burn completely out as the natural draft of air is allowed with the burning wood. The raw ore is roasted in this process and converted into a partially processed ore that will cling strongly to a magnet. Only small percentages of raw ore will initially stick to a magnet before roasting. After roasting, almost all of it sticks to a magnet. Roasting bums out some of the organic matter in the ore and gets rid of some of the volatile matter associated with raw ore.
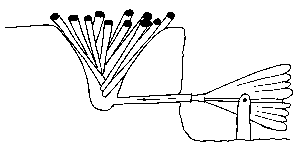
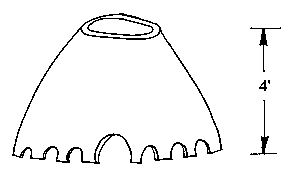
The second step that produces a bloom is to dig a conical hole in the ground and pile it full of pine wood. The wood is set on fire and blown with a bellows to keep it going during the smelting process. The roasted ore is shoveled into the fire and allowed to smelt for a period of several hours while the blooms of iron form. When the fire dies down, after successive stages of replenishing the wood and more roasted ore, small blooms can be extracted. These are then worked and refined in a blacksmith shop by repeated hammering to drive out the slag from the blooms to form useful iron. The second of the papers revealed the results of studying Roman furnaces by Dr. Carl Blair of the University of Wisconsin. He and volunteer graduate students have set up experimental furnaces that exactly duplicate Roman iron smelting procedures based on archaeological studies actually done at Roman furnace sites in Western Europe. Through an analysis of slags, he determined that the Romans did not use flux or limestone in their smelters as we are familiar with in charcoal furnace sites here in America. just ore and charcoal were used in Roman furnaces, although Dr. Blair acknowledged that it would have been a more efficient operation if a limestone flux had been used by the Romans. His experiments indicated that no bellows or blowing devices were used in these furnaces, but that a high furnace stack of at least 2.8 meters had to be used to induce an air flow through the fire. Anything lower would not induce enough draft to take the furnace reaction to smelting completion. The third presentation on iron was given by Dr. Nikolass J. van der Merwe, a professor of Dutch descent who resides in South Africa. He had studied some of the methods of African tribesmen who smelt iron from ore. The technical details are similar to the first two methods with only raw ore and charcoal used with no limestone fluxing added. Flux seems to have two functions in modern practice: 1) It combines with the impurities in the ore and helps to purify the iron and 2) it helps to liquefy and carry off, with greater viscosity, the earthy matter and impurities in the ore. The African methods he studied also had two stages of smelting the iron, as did the Norwegian method. A low but large-diameter furnace is built and filled with charcoal and raw ore. Since the furnace is low, many air holes have to be placed at the base of the furnace to allow air to circulate in the fire and keep it burning. This arrangement is almost directly opposite from the Roman practice but, presumably, the many additional air holes eliminate the need for a high furnace stack. The African-style furnace is very slow burning, but reduces the ore to a very crude bloom at the end of its burning period. The crude bloom produced is pulled from the cold ashes and broken up for further refining in a secondary forge that is considered as part of the smelting process. Once a refined bloom is pulled from the smaller secondary fire, it is forged and consolidated into usable iron from which the tribesmen forge plow points and hoes.
The information presented here on the African furnace is not complete because there are many details and subtleties left out that are necessary to know and experiment with before iron smelting can be successfully done. But these are the major steps that have a remarkable similarity in the processes. One of the final pieces of information Dr. Blair related in a private conversation was that most people who attempt to smelt iron from its ore in their backyard almost always blow the fire much too hard to complete a successful smelting of a bloom of iron in the direct method. A symposium or seminar such as this should be interesting for any blacksmith or farrier who seeks to understand his profession better. It is true we don't have the in-depth historical or scientific background, in most cases, to understand all of the information presented at one of these things, but we do have a unique knowledge about our metal that is badly needed by the scientists and archaeologists who operate at this intellectual level. It was obvious to me, who was more or less an "outsider" at this event, that very few in attendance understood forge welding, where the hammer meets the iron. That one aspect alone seems to me to be a tremendous handicap for anyone seeking to understand the past as it relates to iron. We blacksmiths have an emotional tie to iron that scientists and lab technicians do not understand; that is their loss. Return to the Blacksmithing Articles listing page. Return to the ANVIL Online Table of Contents for March, 1998.
|